Ultrafast Motion of protozoa
unravelling the secrets behind the ultrafast movements of ciliated protists, powered by calcium.
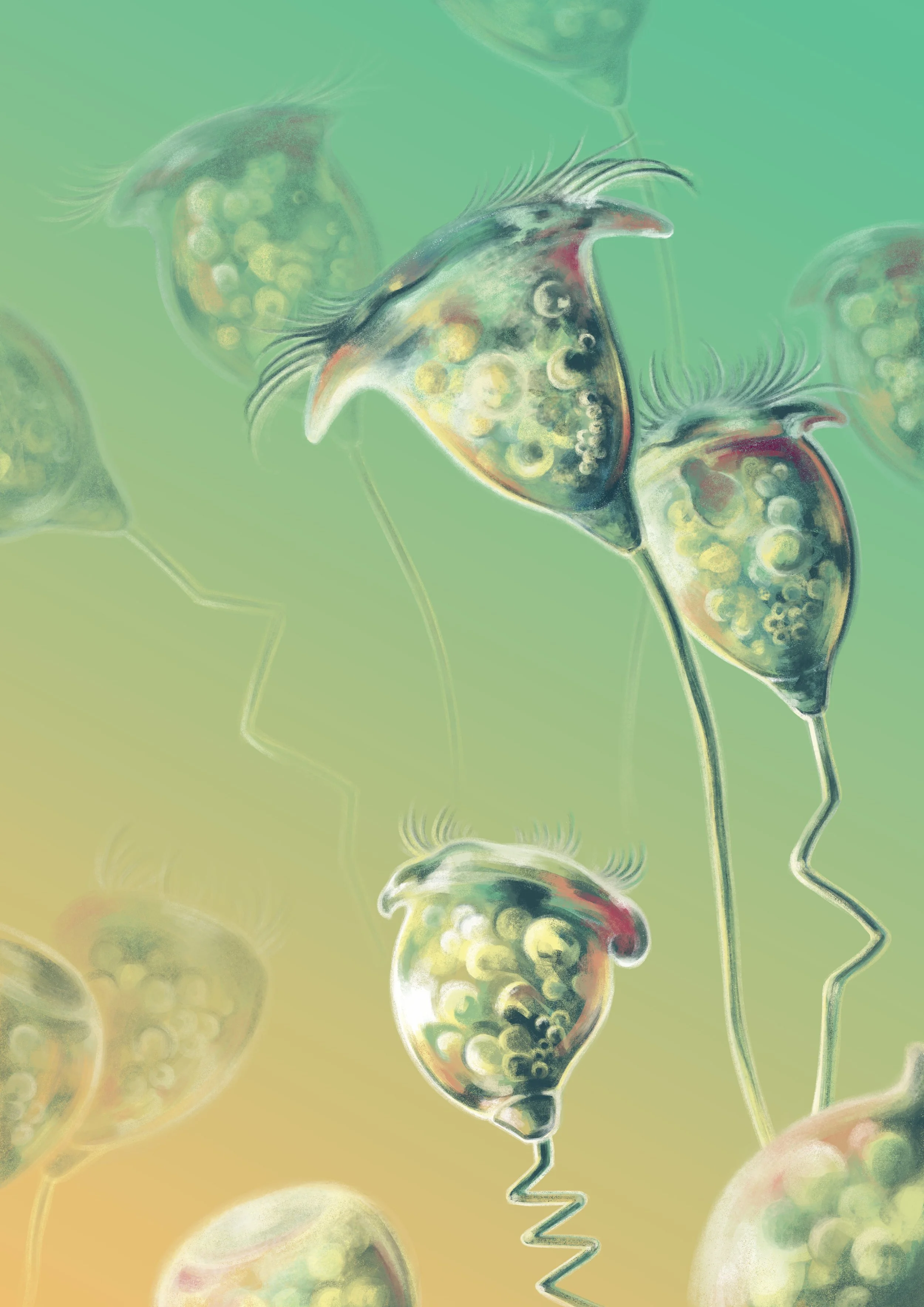
Ultrafast contracting ciliates. Credit: Bhamla Lab. Illustrated by Franz Anthony
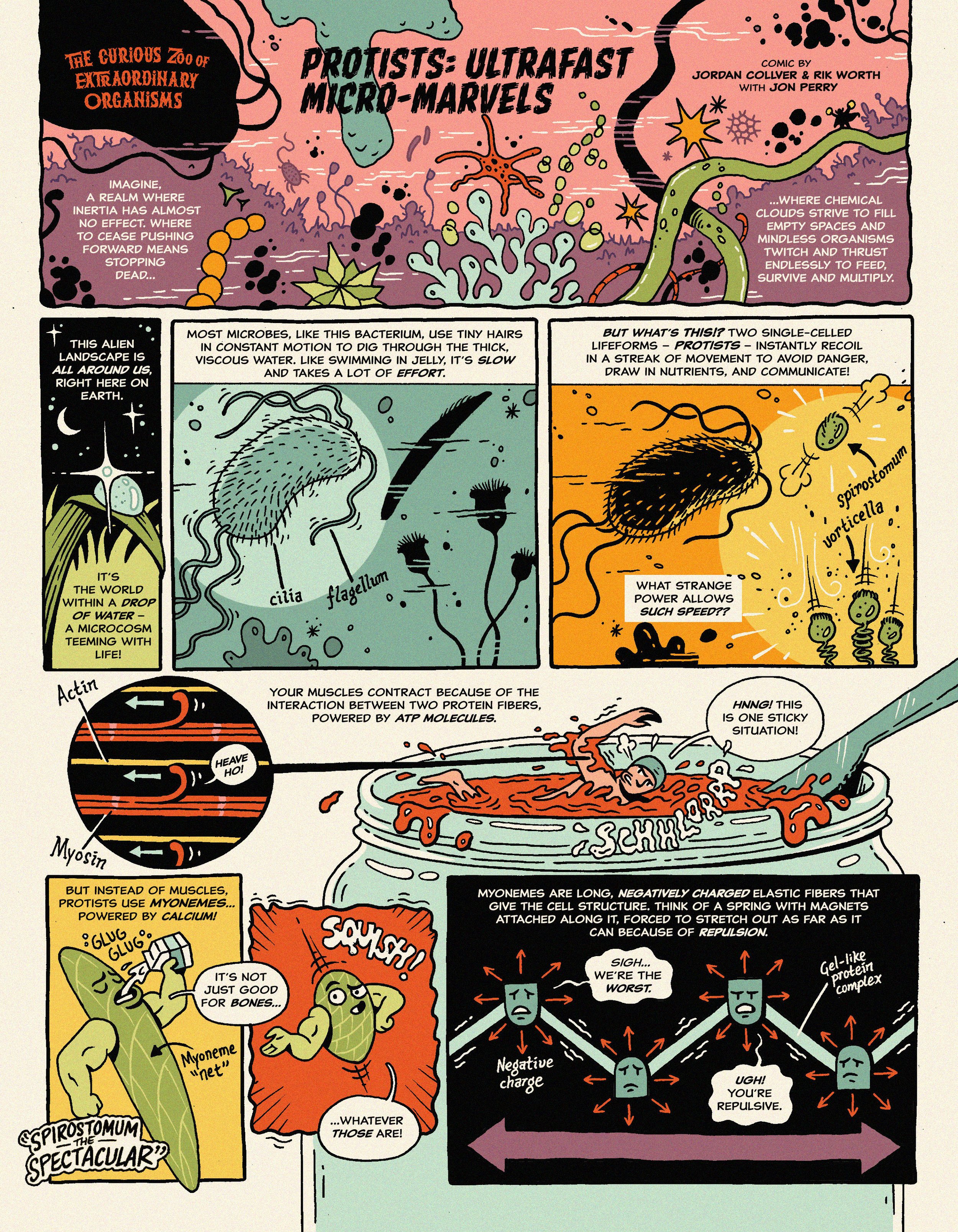
Some single-celled organisms generate ultrafast motion using supramolecular springs or `myonemes’ powered by calcium ions!
Peer into a droplet of fresh pond water under a microscope, and you'll witness a surprising spectacle: microorganisms changing shape faster than a blink of an eye. These ciliated protists have evolved the ability to contract ultrafast, a skill they use for a variety of tasks, from nutrient mixing to predator evasion and even hydrodynamic communication with neighboring organisms.
Scientists have documented these ultrafast motions across numerous species. Yet, our understanding of the mechanisms driving these speedy feats remains limited. The challenge lies in the diversity of these organisms, each boasting different body plans and sizes. The question then arises: Can we find a simple, general physical description that applies across this diverse spectrum of species? If we can, it would pave the way for mimicking this process in synthetic biological systems, potentially allowing us to control mechanical forces at subcellular scales.
“When we first started studying these organisms, we thought it was necessary to account for a lot of details of their specific body plans to explain their contraction speeds. It was satisfying to realize that we can come up with a mathematical description that is very minimal, but which still seems to quite accurately describe these motions.”
A colony of bell-shaped, ciliated protists attached to organic debris that can contract rapidly. On the cover of PNAS (2023). Image credit: Karl Gaff.
Our lab, in collaboration with colleagues from the University of Chicago, North Carolina State, Drake University, and the University of San Francisco, sought to unravel the mechanics behind these contractions. We developed a simple physical model and compared its predictions to experimental measurements of ultrafast contraction in two distinct species of ciliated protists (Spirostomum and Vorticella).
The 3rd plate from Ernst Haeckel's ''Art Forms in Nature'' (1904), depicting organisms classified as Ciliata. (Wikimedia)
Major questions
How do calcium ions drive ultrafast contractions in ciliated protists?
What role do myonemes play in these contractions?
Can a unifying model describe contraction dynamics across different species?
What are the potential applications in synthetic biology?
What we’ve discovered
A Unified Model for Ultrafast Contraction
Our team has developed a minimal mathematical model that accurately describes the ultrafast contraction observed in two species of ciliated protists. This model, which is applicable across species, captures the effect of a traveling calcium wave on an elastic medium in a dissipative environment.
Three Distinct Dynamic Regimes
Our model reveals three distinct dynamic regimes, differentiated by the rate of chemical driving and the importance of inertia. Each regime exhibits unique scaling behaviors and kinematic signatures, providing a comprehensive understanding of the contraction dynamics.
The Calcium Latch Mechanism
Our study sheds light on the unique mechanism of contraction in these protists, which is driven by calcium ions, not ATP. This calcium latch mechanism initiates contraction and then ATP is used to reset the process, offering a new perspective on cellular contraction dynamics.
Implications for Synthetic Biology:
The insights gained from our study not only enhance our understanding of calcium-powered myoneme contraction in protists, but also pave the way for the rational design of ultrafast bioengineered systems. The principles uncovered could inform the development of active synthetic cells, opening new avenues in the field of synthetic biology.
Read the paper
A unified model for the dynamics of ATP-independent ultrafast contraction. (2023)
SEE THE COMICS